China CNC Milling » Blog » Study on the Performance Differences Between NylonPA66 and PA6T Materials
FAQ
What materials can you work with in CNC machining?
We work with a wide range of materials including aluminum, stainless steel, brass, copper, titanium, plastics (e.g., POM, ABS, PTFE), and specialty alloys. If you have specific material requirements, our team can advise the best option for your application.
What industries do you serve with your CNC machining services?
Our CNC machining services cater to a variety of industries including aerospace, automotive, medical, electronics, robotics, and industrial equipment manufacturing. We also support rapid prototyping and custom low-volume production.
What tolerances can you achieve with CNC machining?
We typically achieve tolerances of ±0.005 mm (±0.0002 inches) depending on the part geometry and material. For tighter tolerances, please provide detailed drawings or consult our engineering team.
What is your typical lead time for CNC machining projects?
Standard lead times range from 3 to 10 business days, depending on part complexity, quantity, and material availability. Expedited production is available upon request.
Can you provide custom CNC prototypes and low-volume production?
Can you provide custom CNC prototypes and low-volume production?
Hot Posts
Polyamide (nylon) is a crucial class of synthetic polymer materials. It possesses outstanding mechanical properties, excellent wear resistance, and ease of processing.
These characteristics give it a significant position in modern industry.
Among them, polyhexamethylene adipamide (PA66) and polyphthalamide (PA6T) stand out as two high-performance engineering plastics within the nylon family.
Their unique chemical structures and performance characteristics have established them as core materials in high-end sectors such as automotive, electronics, and aerospace.
However, as industrial technology demands increasingly sophisticated material properties, the distinct characteristics and application scenarios of these two polymers have gradually become focal points of research.
PA66 is a semi-crystalline polymer synthesized through the polycondensation of adipic acid and hexamethylene diamine.
It features a high melting point, excellent mechanical strength, and strong chemical resistance.
However, its high moisture absorption leads to insufficient dimensional stability, limiting its use in precision environments.
PA6T is synthesized from terephthalic acid and hexamethylene diamine.
Its elevated melting point and high heat deflection temperature give it significantly enhanced rigidity and dimensional stability in high-temperature environments.
Additionally, its moisture absorption is only 1/4 to 1/3 that of PA66, markedly reducing performance fluctuations caused by humidity changes.
PA66, with its superior flowability and rapid crystallization rate, is suitable for thin-wall part processing but has a narrower processing window.
PA6T, optimized through molecular structure design, combines high flowability with low warpage characteristics.
These properties make it particularly suitable for thin-wall molding of precision electronic components.
Both PA66 and PA6T are representative nylon materials, yet they exhibit significant differences in performance and applications.
This paper compares the physical properties of PA66 and the high-temperature-resistant nylon PA6T.
It analyzes their distinct mechanical properties and water resistance.
Based on these differences, the study provides application scenario validation and theoretical guidance.
Experimental Section
Primary Raw Materials
Standard nylon, PA66 (containing 30% glass fiber by mass), commercially available;
High-temperature-resistant nylon, PA6T (containing 30% glass fiber by mass), commercially available.
Primary Equipment and Instruments
Experimental injection molding machine, WZS10D, Shanghai Xinshuo Precision Machinery Co., Ltd.;
Electric forced-air drying oven, WG9140AB, Shenzhen Jiangcheng Instrument Co., Ltd.;
Plastic injection molding machine, MA1600Ⅲ/570, Haitian Plastic Machinery Group Co., Ltd.;
Hydraulic testing machine, JJHBT-21015, Chengde Jinjian Testing Instrument Co., Ltd.;
Composite impact testing machine, HIT-2492, Chengde Jinjian Testing Instrument Co., Ltd.;
Electric Heating Constant Temperature Drying Oven, SC101-3A, Yuyao Ditang Town Thermoplastic Spraying Equipment Factory;
Differential Scanning Calorimeter, DSC3+, Mettler-Toledo GmbH;
Electronic Balance, FA2004, Shanghai Youke Instrument Co., Ltd.;
Universal Testing Machine, XWW-20T, Chengde Jinjian Testing Instrument Co., Ltd.
Sample Preparation
1. Water Absorption Sample Preparation
Place PA66 raw material into an electric heating constant-temperature drying oven.
Set the material temperature to 110°C and dry for 4 hours.
After drying, injection mold the sample on an injection molding machine.
Injection molding temperature: 280°C. Sample dimensions: Length (60±2) mm, Width (60±2) mm, Thickness (2.05±0.05) mm.
Molding-finished samples are used for water absorption rate testing.
PA6T samples are prepared using the same method, with the following differences:
– Oven temperature set to 120°C for 6 hours drying
– Injection molding temperature: 350°C
2. Tensile Specimen Preparation
Following the drying and injection molding procedures described in the literature, PA66 and PA6T raw materials were injection molded into tensile test bars using an injection molding machine.
These bars were used for tensile property testing and conformed to the Type 1A dimensions specified in GB/T 1040.2-2022 “Determination of Tensile Properties of Plastics—Part 2: Test Conditions for Molded and Extruded Plastics.”
3. Impact Specimen Preparation
Following the drying and injection molding procedures described in the document, engineers molded PA66 and PA6T raw materials into simply supported beam impact specimens.
They used an injection molding machine to perform the injection molding process.
Engineers used these specimens for impact performance testing.
The specimens conformed to the Type A notch dimensions specified in GB/T 1043.1-2008, “Determination of Impact Performance of Plastics—Part 1: Non-instrumented Impact Test.”
4. Blast Sample Preparation
PA66 and PA6T raw materials were separately injection molded into pipe fittings to study the injection molding processes of both materials (see Figure 1).
Technical parameters are listed in Table 1.

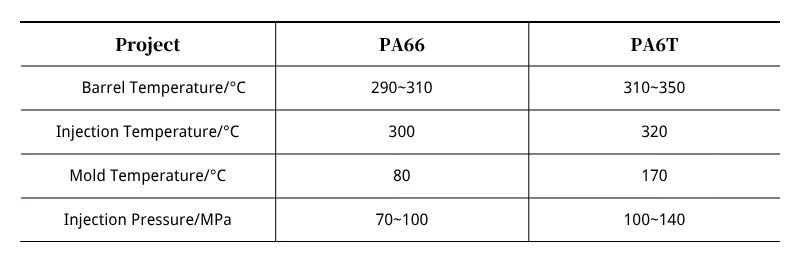
As shown in Table 1: When injection molding parts, PA66 and PA6T exhibit significant differences in injection temperature and mold temperature.
PA66 requires relatively lower injection temperature, mold temperature, and injection pressure.
Attention must be paid to ensuring PA6T material does not remain in the barrel for too long, as this increases the risk of degradation.
Compared to PA6T, PA66 is relatively simpler to process in injection molding.
Testing and Characterization
1. Material Melting and Crystallization Temperature Testing
Following GB/T 19466.3-2004 “Plastics—Differential Scanning Calorimetry (DSC) Part 3:
Determination of Melting and Crystallization Temperatures and Heat of Formation,” cut sample pieces with a scalpel and place them into lightweight aluminum crucibles.
Control the sample quantity between 5–10 mg, accurate to 0.1 mg.
Cover the crucible with its lid and puncture a small hole using a DSC-specific needle.
This allows nitrogen gas to enter the crucible and contact the sample, balancing the internal and external air pressure.
Gently pick up the crucible with tweezers for sample loading.
Turn on the nitrogen gas valve, set the flow meter to 50 mL/min, and select the appropriate testing software.
The maximum temperature is typically set 30K above the sample’s melting point.
When the test is complete, the resulting graph displays time and temperature on the horizontal axis.
The vertical axis shows the heat flux difference between the sample crucible and the reference crucible.
Analysis of the spectrum yields the primary melting peak temperature, crystallization peak temperature, and secondary melting peak temperature.
Researchers commonly adopt the secondary melting peak temperature as the material’s melting temperature.
They can also determine the crystallization peak temperature from the spectrum.
2. Material Water Absorption Test
Per BS EN ISO 62:2008 “Determination of Water Absorption of Plastics,” first place the injection-molded parts in an oven at (50.0±2.0)°C for 24 hours drying, then cool to room temperature in a desiccator.
Weigh each sample using an electronic balance to an accuracy of 0.1 mg. Repeat the preceding steps until the sample’s mass change remains within ±0.1 mg.
Place the sample into a container filled with distilled water maintained at (23.0 ± 1.0)°C.
After immersion for (24 ± 1) hours, remove the sample and promptly wipe the surface moisture with a clean, dry cloth.
Weigh each sample again to an accuracy of 0.1 mg. Weighing must be completed within 1 minute after removing the sample from the water.
Calculate the water absorption mass fraction of each sample relative to its initial mass, i.e.,
.jpg)
Where: c is the water absorption mass fraction of the specimen;
m2 is the mass of the specimen after immersion; m₁ is the mass of the specimen after drying prior to immersion;
m3 is the mass of the specimen after immersion and final drying.
Test results are expressed as the arithmetic mean of three results obtained under identical exposure conditions.
3. Tensile Property Testing
Place one portion of the injection-molded sample strips in a constant temperature and humidity chamber at 23°C and 50% relative humidity for 16 hours of pre-treatment.
Place another portion in a hydrostatic water tank at 95°C for 1000 hours of boiling.
Following GB/T 1040.1-2018 “Determination of Tensile Properties of Plastics – Part 1:
General Principles” and GB/T 1040.2-2022 “Determination of Tensile Properties of Plastics – Part 2:
Test Conditions for Molded and Extruded Plastics,” tensile properties were tested using a universal testing machine under the following conditions: Sensor range: 5000 N; Tensile speed: 50 mm/min.
4. Impact Performance Testing of Simply Supported Beams
One portion of the injection-molded test specimens was pretreated for 16 hours in a constant temperature and humidity chamber at 23°C and 50% relative humidity.
The other portion was boiled for 1000 hours in a hydrostatic water tank at 95°C.
Following GB/T 1043.1-2008 “Determination of Impact Strength of Plastics – Part 1: Non-instrumented Impact Test,” the specimens underwent cantilever beam impact testing using a composite impact testing machine.
Test conditions: pre-lift angle of 114°, pendulum energy of 5J.
5. Burst Performance Testing
Researchers conducted burst pressure testing on a portion of the injection-molded PA66 and PA6T pipe fittings.
This testing followed GB/T 15560-1995, “Hydraulic Instantaneous Burst and Pressure Resistance Test Methods for Plastic Pipes for Fluid Conveyance.”
Another batch of components was immersed in a static hydraulic water tank at 95°C for 1000 hours before undergoing burst pressure testing.
Instantaneous burst tests were conducted using a static hydraulic testing machine with Class A sealing heads at 20°C.
Pressure was applied uniformly and rapidly to cause specimen rupture within 60–70 seconds. The burst pressure, time, and failure mode were recorded.
Results and Discussion
Melting Temperature and Crystallization Temperature
Melting temperature and crystallization temperature were measured using a differential scanning calorimeter (DSC).
For PA66 samples, the temperature range selected for testing was 25–300°C.
For PA6T samples, the temperature range selected for testing was 25–360°C.
Test results indicate: PA66 exhibits a melting temperature of approximately 263°C, while PA6T shows a melting temperature of approximately 314°C.
PA66 demonstrates a crystallization temperature of approximately 225°C, and PA6T exhibits a crystallization temperature of approximately 272°C.
The melting and crystallization temperatures of PA6T parts are elevated by 51K and 47K, respectively, compared to PA66.
This difference arises because PA66’s molecular structure forms primarily through the polycondensation of adipic acid and hexamethylenediamine.
In contrast, PA6T forms through the polycondensation of terephthalic acid and hexamethylenediamine.
The molecular structural variations between these two nylon materials contribute to differences in melting and crystallization temperatures.
They also lead to distinctions in other performance characteristics.
Water Absorption Rate
Water absorption rate testing reveals that PA66 components exhibit a water absorption rate of 3%, while PA6T components demonstrate a rate of only 0.5%.
Analysis indicates that PA66 is synthesized through the polycondensation of hexamethylene diamine and adipic acid.
This results in molecular chains rich in methylene (-CH₂-) units, forming relatively long and flexible fatty chains.
Although the amide bond (-NH-CO-) exhibits strong polarity, the flexibility of the long fatty chains results in relatively loose molecular chain packing.
This allows hydrophilic regions (amide bonds) to be more readily exposed and form hydrogen bonds with water molecules, leading to higher water absorption.
PA6T components exhibit lower water absorption because PA6T is synthesized through the polycondensation of hexamethylene diamine and terephthalic acid.
The incorporation of benzene rings into its basic backbone significantly reduces molecular chain polarity, forming a polyamide resin with low water absorption.
This structural modification results in PA6T having lower water absorption than PA66, meaning PA6T demonstrates superior water resistance.
Tensile Properties
Tensile tests were conducted on specimens before and after water immersion.
PA66 exhibited an initial tensile strength of 140 MPa, which decreased to 105 MPa after boiling, representing a 25% reduction rate.
PA6T exhibited an initial tensile strength of 220 MPa, which decreased to 195 MPa after boiling, representing an 11% reduction.
Since PA66 is synthesized via the polycondensation of hexamethylene diamine and adipic acid, its molecular chains contain a high density of hydrogen bonds.
These hydrogen bonds confer excellent mechanical strength and toughness.
PA6T incorporates aromatic rings, conferring higher heat resistance and rigidity, hence its initial tensile strength exceeds that of PA66.
After boiling, PA66‘s higher water absorption allows water molecules to form hydrogen bonds with amide groups, weakening existing intermolecular hydrogen bonding.
This “plasticizing effect” reduces PA66‘s rigidity and strength.
PA6T, containing aromatic rings, experiences minimal disruption to its molecular structure by water molecules and exhibits lower water absorption.
Consequently, its tensile properties decline less significantly than PA66‘s after water immersion.
Impact Performance of Simply Supported Beams
Impact tests on simply supported beams were conducted on samples before and after water immersion.
PA66 exhibited an initial impact strength of 95 kJ/m², which decreased to 38 kJ/m² after boiling, representing a 50% reduction.
PA6T showed an initial impact strength of 40 kJ/m², dropping to 32 kJ/m² after boiling, a 20% reduction.
PA6T exhibits lower initial impact strength than PA66 due to its molecular structure containing more rigid benzene rings.
After boiling, PA66‘s significantly higher water absorption rate caused a marked decline in its impact strength.
Conversely, PA6T‘s lower water absorption resulted in a smaller reduction in impact performance compared to PA66 after water immersion.
Burst Performance
Burst performance tests were conducted on sample strips before and after water immersion.
Prior to hydrostatic testing, PA66 exhibited a burst pressure of 7.7 MPa, while PA6T recorded 5.4 MPa.
After 1000 hours of boiling at 95°C, the burst pressure of PA66 components decreased by 71.4% to 2.2 MPa, whereas PA6T components showed only a 7.4% reduction to 5.0 MPa.
Analysis indicates that PA6T is synthesized via the polycondensation of terephthalic acid and hexamethylenediamine.
This process produces highly symmetrical molecular chains, which promote high crystallinity and rapid crystallization.
In contrast, PA66 exhibits less symmetrical molecular structures and lower crystallinity, leading to slower crystallization rates.
During injection molding, PA6T‘s excessively rapid crystallization prevents complete fusion at the parting line, creating weak points.
Burst testing rapidly identifies the component’s weakest location, explaining why PA6T exhibits lower burst pressure than PA66.
Additionally, the PA66 molecular chain contains numerous amide groups.
These polar groups form strong hydrogen bonds through mutual repulsion, resulting in significant intermolecular forces.
Consequently, the material exhibits higher strength and toughness, enabling it to withstand greater burst pressure.
PA6T, however, is synthesized through the polycondensation of terephthalic acid and hexamethylenediamine. Its molecular chains contain benzene ring structures.
While these rings enhance rigidity, the reduced number of amide groups results in weaker intermolecular hydrogen bonding.
Consequently, PA6T exhibits lower toughness than PA66 and cannot withstand higher burst pressures.
After boiling in water, water molecules penetrate the PA66 material and form hydrogen bonds with the amide groups.
This weakens the original hydrogen bonding between molecular chains, leading to a significant reduction in burst pressure.
Conclusion
The study systematically compared the melt/crystallization behavior, moisture absorption characteristics, and mechanical properties of PA66 and PA6T materials.
Through accelerated aging in a high-temperature water environment at 95°C for 1000 hours, it validated the reliability differences between the two materials in thin-walled pressure-bearing components.
Results indicate: PA66 exhibits a high water absorption rate of 3% due to its aliphatic chain structure.
After water boiling, its tensile strength, impact strength, and burst pressure degraded by 25%, 50%, and 71.4% respectively, rendering it unsuitable for long-term service in high-temperature aquatic environments.
In contrast, PA6T exhibits a water absorption rate of only 0.5% due to the rigidity-hydrophobicity synergistic effect introduced by its benzene rings.
Under identical conditions, its performance degradation remains ≤20% across all parameters, with a burst pressure retention rate exceeding 90%, demonstrating outstanding resistance to hot water aging.
For applications in dry or low-temperature water environments, conventional PA66 remains the preferred choice due to its simple injection molding process, stable mechanical properties, and high overall cost-effectiveness.
However, when subjected to prolonged high-temperature water exposure, conventional PA66 suffers significant performance degradation due to poor hydrolysis resistance, making high-temperature PA6T the superior material.
Despite process challenges such as high melt temperature, narrow injection window, and slightly lower initial burst pressure, PA6T‘s superior dimensional stability and durability make it the material of choice for high-temperature water-contact components.
Future efforts will focus on optimizing PA6T crystallization rates, enhancing weld strength, and developing high-flow grades to further expand its application boundaries in automotive thermal management systems, high-end home appliances, and core plumbing components.